What Do Sacrificial Anodes Do?
When metals are in water, they create an electrical charge. When two different metals are in contact in water, they form a small electric circuit. The less noble metal (like a bronze propeller) becomes the anode, and the more noble metal (like a stainless steel shaft) becomes the cathode. This setup can cause the anode to corrode.
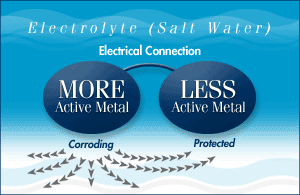
To protect the metal parts of your boat, you can use a sacrificial anode, which is a third metal that is more active than the other two. This metal will corrode first, protecting the other metals from rusting. Aluminum is a great choice for sacrificial anodes because it provides more protection and lasts longer than zinc. It works well in both fresh and salt water, making it safe for all types of applications.
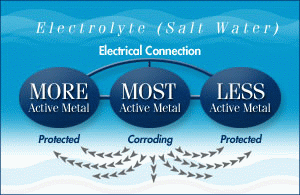
What Metals Are Sacrificial Anodes Made From?
Sacrificial anodes are usually made from zinc, aluminum, or magnesium. These metals have different properties:
- Magnesium: Generates -1.6 volts
- Aluminum: Generates -1.1 volts
- Zinc: Generates -1.05 volts
The larger the voltage difference between the anode and the metal to be protected, the better the protection. For example, using zinc to protect a bronze propeller provides a -0.75 volt difference. Aluminum anodes increase this to -0.8 volts, and magnesium anodes increase it to -1.3 volts. However, using magnesium can sometimes lead to overprotection.
The lifespan of an anode also varies. If a zinc anode lasts 100 days, a magnesium anode of the same size would only last 30 days. An aluminum anode would last between 130 and 150 days.
Choosing the Right Anode Material for Your Boat
The type of boat you have will determine the best anode material:
- Inboard Boats: Use zinc or aluminum anodes. These boats generally have bronze and stainless steel parts.
- Sterndrives and Outboard Motors: Use aluminum anodes. Zinc anodes may not provide enough protection, and using magnesium can cause overprotection.
- Freshwater Boats: Aluminum anodes are recommended over zinc because zinc can become inactive over time in freshwater.
Factors That Increase Corrosion
Several factors can increase corrosion, including:
- The voltage difference between metals.
- Saltier water.
- Higher water temperatures.
- Pollution, such as acid rain, which increases water conductivity.
When to Replace Sacrificial Anodes
Replace anodes at least once a year or when they have corroded to half their original size. Some anodes come with a wear indicator that shows a red spot when it’s time to change them.
Installing New Anodes
When installing new anodes:
- Ensure they make good electrical contact with the metal being protected.
- Clean the metal surface before attaching the anode.
- Do not paint the anodes, as this will prevent them from working.
Additional Tips for Protecting Your Sterndrive
- Keep the paint on engines and sterndrive units in good condition.
- Leave the sterndrive unit immersed in water so the anodes can work.
- Avoid using anti-fouling paint containing copper or mercury, as it increases corrosion.
- Do not mix zinc anodes on the hull with aluminum anodes on the drive, as this can lead to improper protection.
How Do Keeper (Aluminum) Anodes Protect Aluminum Outdrives?
Keeper anodes are made from a combination of aluminum, zinc, and indium. This mix prevents the anode from forming an oxide coating, making it more effective at protecting aluminum outdrives.
By understanding how sacrificial anodes work and choosing the right material for your boat, you can protect your metal parts from corrosion and extend their lifespan.
